The National Agency for Drug Safety (ANSM) announced in a statement on February 15 that a national pharmacovigilance survey had been opened in September 2016 on all medicines containing docetaxel, a generic taxoterexine drug, after reporting of several deaths.
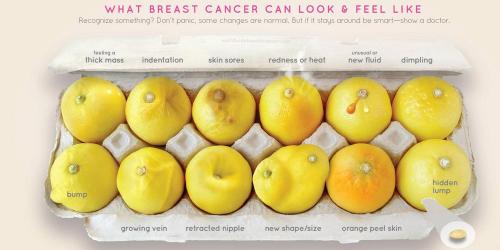
It is usually prescribed in cases of breast cancer (adjuvant in operable breast cancer, locally advanced or metastatic breast cancer), lung, prostate, aerodigestive tract or gastric cancer.
Updated July 6, 2017
At the end of its pharmacovigilance survey, the ANSM, in agreement with the National Cancer Institute (INCa), announced in a statement issued on July 5, 2017 that the recommendation to temporarily avoid docetaxel in the treatment of Non-metastatic infiltrating breast cancer was lifted.
The health authority says the investigations "do not show an increase in the frequency of serious adverse events and deaths related to this molecule." According to her, cases of serious adverse events and deaths "remain rare during the twenty years of marketing of this drug, of the order of 1 per 10,000 patients exposed to docetaxel."
It also states that "docetaxel and paclitaxel are important therapeutic options and have reduced mortality in a large number of cancers, some of which have no alternative, such as many other cancer drugs. Adverse effects and their use entails risks INCa and ANSM consider that these must be taken into account and anticipated, but must not deprive patients of these effective drugs. "
ANSM and INCa also wish to raise the awareness of health professionals, as well as patients, about certain pathologies (neutropenia, enterocolitis, neuropathies and hypersensitivity reactions) that could constitute potentially serious risks and how to manage them. .
Several deaths of breast cancer patients at the origin of the investigation
The pharmacovigilance survey was conducted following the deaths of five women between the ages of 46 and 73 between August 2016 and February 2017 as a result of enterocolitis, an inflammation of the small intestine and colon.
Suffering from breast cancer, they were treated with docetaxel "alone or in combination, adjuvant or neo-adjuvant breast cancer," explained a first statement from the ANSM.